The Clostridia are a member of the family of Firmicutes. They are spore-forming, gram-positive, obligate anaerobes. The majority of the genus are saprophytic organisms that ferment plant polysaccharides and are found in many places in the environment, most notably the soil. They also form part of the normal gastrointestinal flora in humans.
The genus does, however, contain some pathogens that produce tissue-destructive and neural exotoxins that are responsible for disease manifestations. Clostridia typically become pathogenic when tissue oxygen tension and pH are low, for example, in ischaemic or devitalised tissue.
Clinically important clostridial infections that can present to the Emergency Department include:
- Clostridium difficile associated diarrhoea (CDAD)
- Clostridial gas gangrene
- Tetanus
Clostridium difficile associated diarrhoea
Clostridium difficile is present in the gut of approximately 3% of healthy adults. (2012 UK HPA estimates). Clostridium difficile associated diarrhoea (CDAD) often follows the use of broad-spectrum antibiotics, which alter the normal bacteria flora of the bowel and allow Clostridium difficile to proliferate. The mucosa of the large intestine becomes inflamed and bleeds, taking on the characteristic ‘pseudomembranous appearance’. In March 2015, NICE published the following advice regarding the risk of Clostridium Difficile infection due to the use of broad-spectrum antibiotics.
Clostridium Difficile 027 is a hyper-virulent strain that is increasing in incidence mainly in North America and is also becoming more prevalent in the UK. The main infecting strain in the UK remains Clostridium Difficile 001.
Over 80% of Clostridium difficile infections are reported in people over the age of 65.
The main clinical features of CDAD are:
- Abdominal cramps,
- Severe bloody and/or watery diarrhoea
- Diarrhoea is often offensive smelling
- Fever
Patients can go on to develop toxic megacolon, and the condition can be fatal, especially in frail or elderly patients.
The current gold standard for the diagnosis of Clostridium difficile colitis is cytotoxin assay. This test does have its disadvantages, however, as it is challenging to perform, and results can take up to 48 hours to become available. The most common laboratory test for diagnosing Clostridium difficile colitis is an enzyme-mediated immunoassay that detects toxins A and B. This test has a specificity of 93-100% and a sensitivity of 63-99%.
Stool culture to detect Clostridium difficile is expensive and is not specific for pathogenic strains; it is, therefore, not specific for a diagnosis of CDAD. Sigmoidoscopy is not used routinely but is sometimes performed if a rapid diagnosis is required or if the patient has an ileus. The characteristic ‘pseudomembranous appearance’ is seen in approximately 50% of patients, and a biopsy can be performed. Abdominal X-ray and CT scanning are also not used routinely but can be helpful in patients with severe disease when severe complications, such as perforation and toxin megacolon, are suspected.
Hand washing is an effective way to prevent the spread of Clostridium difficile. Hands should be washed after using the bathroom and before eating. Hands should ideally be wet with water and plain or antibacterial soap and rubbed together for 15 to 30 seconds. Particular attention should be paid to the fingernails, between the fingers, and the wrists. Hands should be rinsed thoroughly and dried with a single-use towel. Patients and family members are encouraged to remind healthcare providers to wash their hands as well.
Alcohol hand gel is ineffective against Clostridium difficile spores, and handwashing with soap and running water is, therefore, essential for healthcare providers that come into contact with it. The wearing of an apron and gloves and isolation to a side room are important contact precautions but will be ineffective if hand washing is neglected and are secondary in importance.
Oral metronidazole is the current first-line treatment of choice in cases of CDAD. Vancomycin is the second-line agent. Antibiotics do not, however, limit spread from patient to patient.
Clostridial gas gangrene
Clostridium perfringens is the organism most commonly associated with gas gangrene (85-90% of cases), but other species, such as Clostridium septicum and Clostridium novyi, can also be implicated. It is capsulate and produces a range of toxins, of which lecithinase C (alpha-toxin) is the most important and is the cause of gas gangrene.
Gas gangrene typically develops when a devitalised wound becomes contaminated with spores from the environment. The spores germinate, and organisms multiply in the ischaemic conditions, releasing toxins, which can further damage tissues. 60% of all cases are post-traumatic, and the majority of these cases follow road traffic accidents.
The clinical features of gas gangrene usually appear within 24 hours of the injury. Initially, there is oedema and reddening and then blackening of the muscles. The overlying skin has a marbled appearance before turning black and sloughing off. There is usually a foul-smelling serous exudate. Crepitus can often be detected under the skin due to gas production by the Clostridia. Progression to toxaemia and shock is often very rapid.
Treatment with antibiotics alone is ineffective, as they do not penetrate into the ischaemic tissues effectively. Treatment is by debridement and excision of infected, necrotic tissues with adjuvant antibiotic therapy. Hyperbaric oxygen may also be helpful. The condition can be prevented by good management of potentially infected devitalised wounds.
Clostridium perfringens is also an important cause of intestinal infections. The spores survive cooking and, during slow cooling and unrefrigerated storage, germinate to form vegetative cells. It is the third commonest cause of food poisoning in the UK.
Tetanus
Tetanus is an acute disease caused by the action of tetanus toxin (the exotoxin tetanospasmin), released following infection by the bacterium Clostridium tetani. Tetanus spores are present in soil or manure and may be introduced into the body through a puncture wound, burn or scratch. The incubation period is quoted as anywhere between 4-21 days and can occur after several months, but symptoms usually occur within the first 7 days after exposure.
The case-fatality ratio ranges from 10 to 90%; it is highest in infants and the elderly. It varies inversely with the length of the incubation period and the availability of intensive care. Neonatal tetanus is an significant cause of death in many countries in Asia and Africa due to infection of the baby’s umbilical stump.
Approximately 80% of patients develop generalised tetanus. The commonest presenting feature of generalised tetanus is trismus (lockjaw), occurring in approximately 75% of affected individuals. Other clinical features include:
- Facial spasms (risus sardonicus)
- Opisthotonus (characteristic body shape during spasms)
- Neck stiffness
- Dysphagia
- Calf and pectoral muscle rigidity
- Fever
- Hypertension
- Tachycardia
Localised tetanus is a rare form of the disease, occurring in around 1% of affected individuals. Patients have persistent contraction of muscles in the same anatomic area as the injury. It may precede generalised tetanus.
Tetanus-prone wounds include:
- Puncture-type injuries acquired in a contaminated environment and likely therefore to contain tetanus spores, e.g. gardening injuries
- Wounds containing foreign bodies
- Open (compound) fractures
- Wounds or burns with systemic sepsis
- Certain animal bites and scratches – although smaller bites from domestic pets are generally puncture injuries, animal saliva should not contain tetanus spores unless the animal has been routing in soil or lives in an agricultural setting.
High-risk tetanus-prone wounds include any of the above with either:
- Heavy contamination with material likely to contain tetanus spores, e.g. soil, manure
- Wounds or burns that show extensive devitalised tissue
- Wounds or burns that require surgical intervention that is delayed for more than six hours are high risk even if the contamination was not initially heavy.
Any tetanus prone wound should be thoroughly cleaned as part of the initial management.
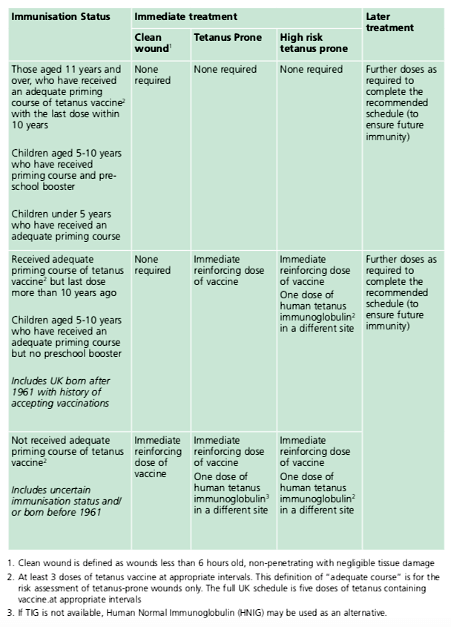
Tetanus prone wounds only require tetanus immunoglobulin (TIG) if:
- The wound is high risk, and an adequate priming course of tetanus vaccine received, but the last dose was more than ten years ago
- The wound is high risk in a child aged 5-10 years who has received an adequate priming course but no preschool booster
- The wound is tetanus prone, and the patient has not received an adequate priming course of tetanus vaccine
- The wound is tetanus prone, and there is uncertain immunisation status and/or the patient was born before 1961
The preventative dose of tetanus immunoglobulin is 250 IU in most cases unless over 24 hours have passed since the injury or the wound is heavily contaminated, then 500 IU should be given.
The immunisation recommendations for clean and tetanus-prone wounds are summarised in the table below:
An intravenous (IV) tetanus immunoglobulin (TIG) product is no longer available in the UK. In the absence of IV-TIG, human intravenous immunoglobulin (IVIG) is currently the recommended treatment for clinically suspected tetanus.
The recommended dose by infusion is 5000 IU for individuals less than 50kg and 10,000IU for individuals over 50kg.
Further reading:
The Green Book Tetanus Overview
Header image used on licence from Shutterstock
Thank you to the joint editorial team of www.mrcemexamprep.net for this ‘Exam Tips’ post.