The amount of water in the body varies with a patient’s age, weight, and sex. Total body water (TBW) accounts for around 60% of an adult male’s total body weight; a normally hydrated 75 kg man will consist of approximately 45 L of water.
There are two major fluid compartments in the human body:
- Intracellular fluid (ICF): This is the water within the cells and accounts for approximately 65% of total body water (30 L of fluid). This is the matrix in which intracellular organelles are suspended, and chemical reactions take place.
- Extracellular fluid (ECF): This is the water outside of the cells and accounts for approximately 35% of total body water (15 L of fluid).
These compartments are separated by the plasma membrane of the cells and differ markedly in terms of the concentrations of the ions dissolved in them.
The extracellular fluid is further divided into three other compartments:
- Interstitial fluid (ISF): This is the tissue fluid found in the spaces between the cells and accounts for approximately 65% of the ECF (10 L of fluid).
- Intravascular fluid: This is the plasma, which is the liquid component of the blood and accounts for approximately 25% of the ECF (3.5 L of fluid).
- Transcellular fluid: This is the final 1.5 L of fluid and comprises intraocular fluid, cerebrospinal fluid, urine in the bladder, and fluid within the lumen of the bowel.
The barrier between the interstitial fluid and the intravascular fluid consists of the walls of capillaries.
Third spacing is the unusual accumulation of fluid in the transcellular space. Examples of third spacing include:
- Pooling of fluids at burn sites
- Ascites
- Pleural effusions
- Fluids leaking from the peritoneal cavity, e.g. in pancreatitis
Intracellular vs extracellular fluid
The composition of ions between the fluid compartments varies, but within any one compartment, electrical neutrality is maintained with the total number of positive charges always being equal to the total number of negative charges.
The most important difference between the ICF and the ECF is the relative concentration of cations (positively charged ions):
- The potassium ion (K+) concentration is much higher in the ICF than in the ECF
- Conversely, the sodium ion (Na+) concentration is much higher in the ECF than in the ICF
- Calcium ion (Ca2+) and chloride ion (Cl–) concentrations are also higher in the ECF.
The two main factors that contribute to the maintenance of the cationic differences between the ICF and the ECF are the activity of the sodium-potassium ATPase (NA-K ATPase) and the Donnan equilibrium.
The approximate concentrations of ions between the three main fluid compartments are summarised in the table below:
| Fluid compartment | Ion concentration (mEq/L) |
|---|---|
| Intracellular fluid (~65%) | Na+ 10 K+ 140 Ca2+ <0.01 Cl- 3-30 HCO3- 9 Protein (-ve) 50 Albumin (-ve) 61-88 |
| Interstitial fluid (~22%) | Na+ 143 K+ 4 Ca2+ 3 Cl- 129 HCO3- 29 Protein (-ve) 1 Albumin (-ve) 0 |
| Plasma (~13%) | Na+ 143 K+ 4 Ca2+ 3 Cl- 108 HCO3- 29 Protein (-ve) 10 Albumin (-ve) 3 |
The NA-K ATPase
The Na-K ATPase is a transporter found in the outer plasma membrane if cells. For every single ATP consumed, it pumps 3 Na+ out of the cell and K+ into the cell, against the concentration gradients.
The Na+ K+-ATPase pump, therefore, maintains the gradient of a higher concentration of sodium extracellularly and a higher level of potassium intracellularly.
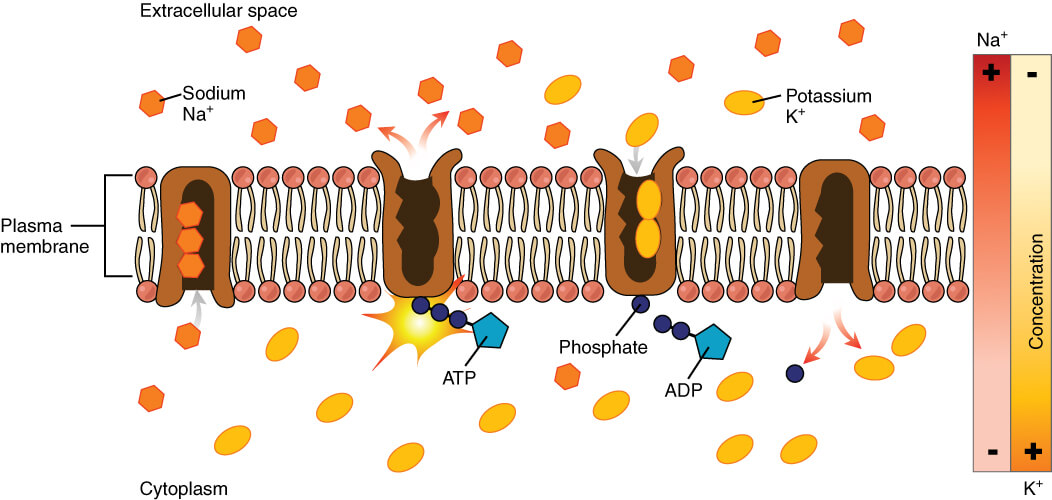
The NA-K ATPase, image sourced from Wikipedia
Courtesy of OpenStax College CC BY-SA 4.0
The Donnan equilibrium
The Donnan equilibrium, or Gibbs-Donnan effect, is the name given to the behaviour of charged particles near a semi-permeable membrane.
It is the state of equilibrium that exists at a semi-permeable membrane when it separates two solutions containing electrolytes, the ions of some of which are able to permeate the membrane, and some are not. An electrical potential develops between the two sides of the membrane, and the two solutions will have different osmotic pressures. With regards to the ICF and ECF, the Donnan equilibrium mainly influences the movement of chloride ions.
Intracellular proteins are negatively charged at physiological pH. Because of their large size, these proteins are unable to cross the plasma membrane, and along with other large anions, such as phosphate, these account for most of the anion content of the ICF.
Chloride ions, however, are small enough to cross the plasma membrane, and these are forced out of the cell by the charge of the fixed proteins and other large anions. The Donnan equilibrium ensures that electrical force driving the chloride ions out of the cell is balanced by the chemical gradient driving them back in.
Interstitial fluid vs plasma
The principle difference between the interstitial fluid and the plasma is the higher protein content of the plasma. Plasma proteins are the only constituents of the plasma that do not cross into the interstitial fluid under normal circumstances.
These proteins exert an osmotic force called the plasma oncotic pressure that almost balances the hydrostatic pressure imposed on the plasma by the action of the heart. This results in a small net movement of water out of the capillaries into the interstitial space. The lymphatic system subsequently absorbs this leakage.
Header image used on licence from Shutterstock
Thank you to the joint editorial team of www.mrcemexamprep.net for this article.






Very nice and brief explanation