Nosocomial infections are defined as those occurring within 48 hours of hospital admission, 3 days of discharge or 30 days of an operation. They affect 1 in 10 patients admitted to hospital. Annually, this results in 5000 deaths with a cost to the National Health Service of a billion pounds. On average, a patient with a hospital-acquired infection spent 2.5-times longer in hospital, incurring additional costs of £3000 more than an uninfected patient. Intensive care units (ICU) have the highest prevalence of hospital-acquired infections in the hospital setting.
Environmental factors
The potential for person-to-person transmission of organisms within hospitals is enormous and is contributed to by numerous environmental factors within the hospital.
These factors include:
- Air supply – pathogens can be transmitted via piped air supplies in theatres and air conditioning (e.g. Legionella pneumophila, tuberculosis, respiratory viruses)
- Water supply – pathogens can be transported to washbasins and showers; Legionella can also colonise redundant areas of pipework
- Food supply – food is usually prepared centrally in the hospital kitchens, and patients are at risk of food-borne infection if hygiene is substandard standards are s
- Fomites – any inanimate object (e.g. vases, fans, viewing boxes) can become contaminated with organisms and act as a vehicles for transmission (fomites).
Patient-centred factors
Hospital inpatients can be more susceptible to infection because of several factors, including:
- Restricted access to washing facilities
- Effects of close patient contact in busy areas (e.g. Emergency Department)
- Contact with contaminated staff clothing (hence the need for ‘bare below the elbows’ approach)
- The patients underlying illness or treatment (e.g. immunosuppression)
- Immobility due to age or co-morbidities
- Ischaemia may make tissues more susceptible to bacterial invasion
Cannulation and central venous catheters
Cannulation is the commonest cause of hospital-acquired bacteraemia. The commonest implicated organisms are Staphylococcus aureus and Staphylococcus epidermidis. The risk increases directly in proportion to the length of time in-situ, and peripheral cannulae should be replaced after 48 hours.
Central venous catheters are a common source of infection and should be suspected as a source of sepsis in any patient that has had a line in situ for a prolonged period (usually longer than a week).
Diagnosis can be difficult, and it is important to note that only 50% of patients with line sepsis have evidence of infection at the insertion site.
The following features are indicative of the vascular catheter as the source of infection:
- Bacteraemia (or fungaemia) in an immunocompetent patient without any underlying disease
- Absence of another identifiable source of infection
- Presence of a vascular catheter (or alternative intravascular device) or the onset of fever
- Inflammation or purulence at the catheter insertion site or along the tunnel
The current recommendation by NICE and the BNF on the treatment of septicaemia related to vascular catheters is to use vancomycin first-line. If Gram-negative sepsis is suspected, especially in the immunocompromised, then a broad-spectrum antipseudomonal beta-lactam antibiotic should be added.
Urinary catheters
Indwelling urinary catheters provide a route for ascending infection into the bladder. The risk of infection can be minimised by aseptic technique when the catheter is handled and inserted.
The commonest organism isolated in catheter-associated bacteraemia is Escherichia coli. Other implicated organisms include Proteus mirabilis and Pseudomonas aeruginosa. This study provides a useful overview of this topic.
For people with a catheter-associated urinary tract infection, consider removing or changing the catheter as soon as possible if it has been in place for longer than 7 days, without delaying antibacterial treatment. An immediate antibacterial prescription should be given, and a urine sample obtained before treatment is taken and sent for culture and susceptibility testing.
If no upper urinary tract infection symptoms are present, the oral first-line recommendations by NICE and the BNF are:
- Amoxicillin (only if culture-sensitive), or
- Nitrofurantoin, or
- Trimethoprim (if low risk of resistance)
If the patient is severely unwell or unable to tale oral treatment, then intravenous therapy with one or a combination of the following should be considered; amikacin, ceftriaxone, cefuroxime, ciprofloxacin, gentamicin, or co- amoxiclav.
Hospital-acquired pneumonia
For the reasons outlined above, hospital-acquired pneumonia is a common problem. The current recommendations by NICE and the BNF on the treatment of hospital-acquired pneumonia are:
- Early-onset infection (less than 5 days after admission to hospital): co-amoxiclav or cefuroxime for 7 days
- Late-onset infection (more than 5 days after admission to hospital): an antipseudomonal penicillin (e.g. piperacillin with tazobactam), a broad-spectrum cephalosporin (e.g. ceftazidime), or a quinolone (e.g. ciprofloxacin)
Control of hospital-acquired infection
Every hospital should have procedures to ensure that infection is not transmitted within its environment. This is generally overseen by a control of infection team, consisting of a consultant microbiologist, specialist nurses and laboratory staff.
Measures taken to control hospital-acquired infection include the following:
- Good clinical practice (basic hygiene measures, hand-washing, ‘bare below the elbows’ approach, staff screening and surveillance)
- Screening and isolation of infected patients (side room facilities with washbasin and separate toilet, alcohol gel, disposable aprons and gloves should be used)
- Respiratory isolation (face masks should be worn by staff and patients when there is a risk of transmission of respiratory pathogens)
- Sharps containers (to protect staff and patients from sharps injuries, these should be sealed and discarded when they are three-quarters full)
- Sterilisation and disinfection (see below)
Effective hand washing
The correct method for hand washing should be known:
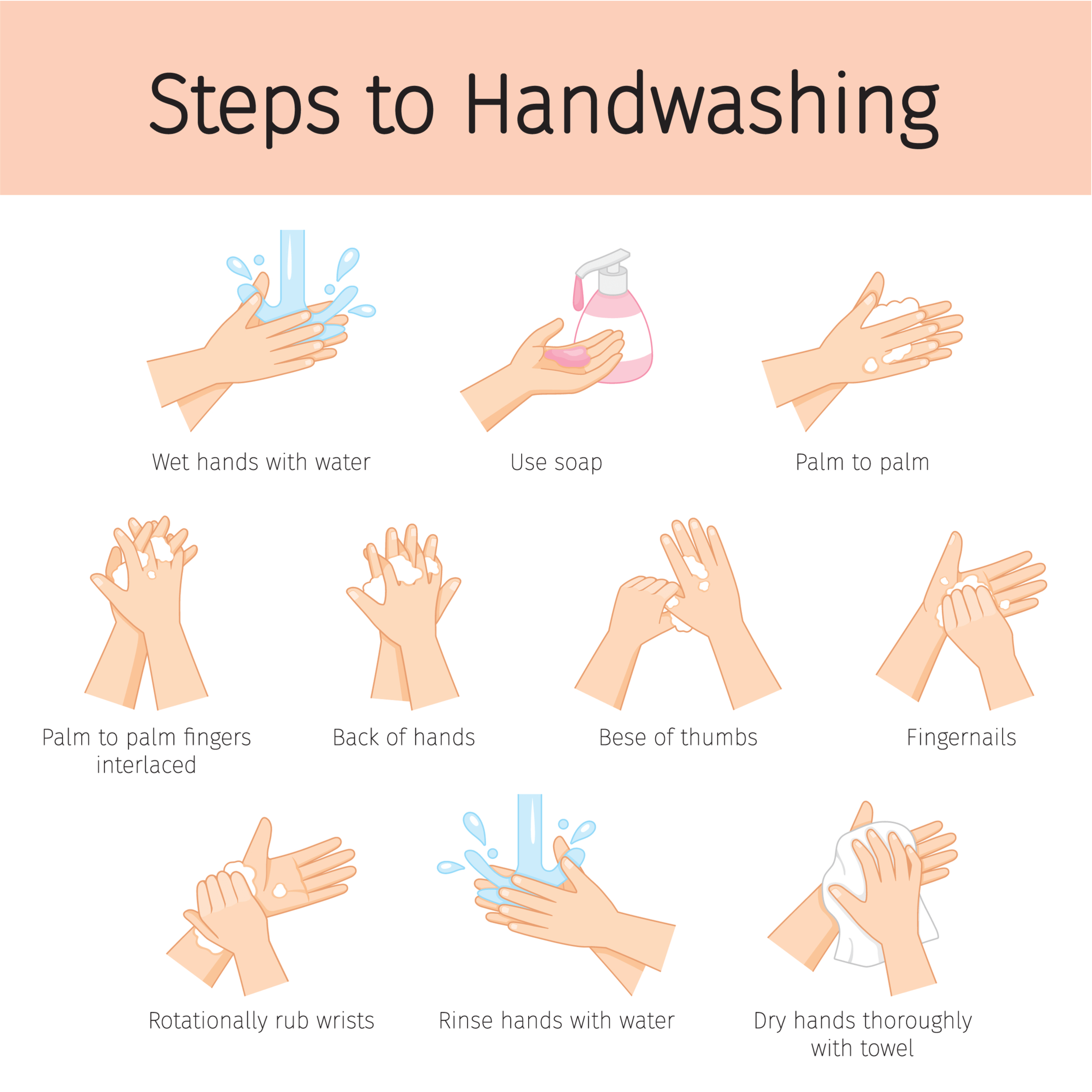
Image adapted from Shutterstock (used on licence)
Sterilisation
Sterilisation inactivates all infectious organisms and is achieved by autoclaving or irradiation.
Autoclaving is the most effective method of sterilising equipment such as surgical instruments and lab equipment to kill harmful bacteria, viruses, fungi, and spores. The autoclaving process takes advantage of the phenomenon that the boiling point of water increases when it is under high pressure. It is performed in a machine known as the Autoclave where high pressure is applied with a recommended temperature of 121°C for 15-20 minutes to sterilise the equipment.
Gamma irradiation sterilisation is performed by exposing the product to a radiation source, typically Cobalt 60 isotope, which decomposes into Nickel 60 isotope, firing off gamma rays in the process. These gamma-rays can penetrate through the entire product, deactivating whatever microorganisms may be present.
Other sterilisation methods include:
- Dry-heat sterilisation
- Paracetic acid sterilisation
- Performic acid sterilisation
- Liquid chemical sterilisation
- Filtration
Disinfection
Disinfection is effective cleaning with chemical disinfectants that can effectively reduce the number of infectious particles. Disinfectants are chemicals that kill or inhibit microbes. Simple washing with soaps or detergents is the most important component in disinfection.
Halogen compounds, such as iodine, are active against bacteria, including spore-bearing organisms, but are relatively slow-acting. Chlorhexidine is also effective against bacteria, especially staphylococci, and can also be used for skin disinfection.
Header image used on licence from Shutterstock
Thank you to the joint editorial team of www.mrcemexamprep.net for this article.






